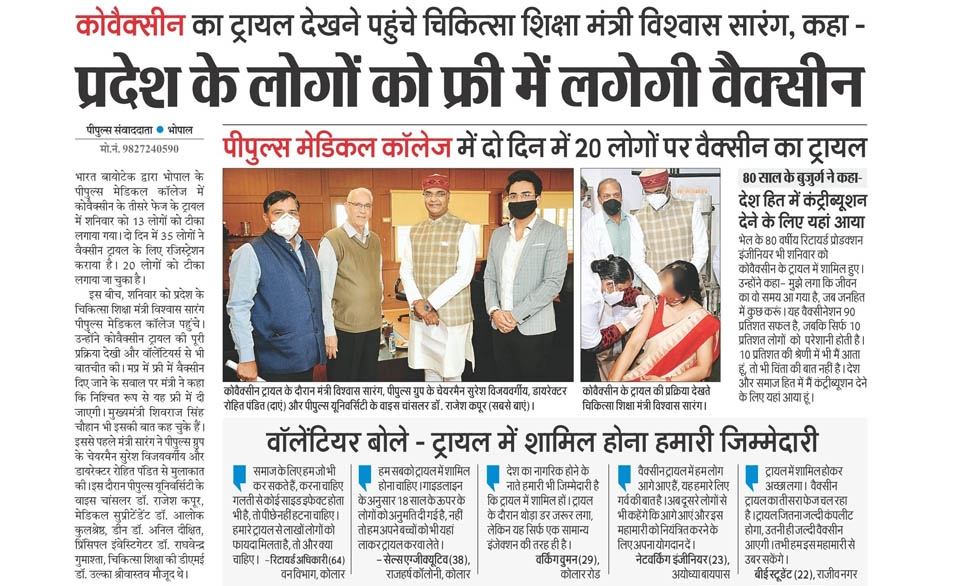
COVAXIN TM – India’s First indigenous COVID-19 Vaccine.
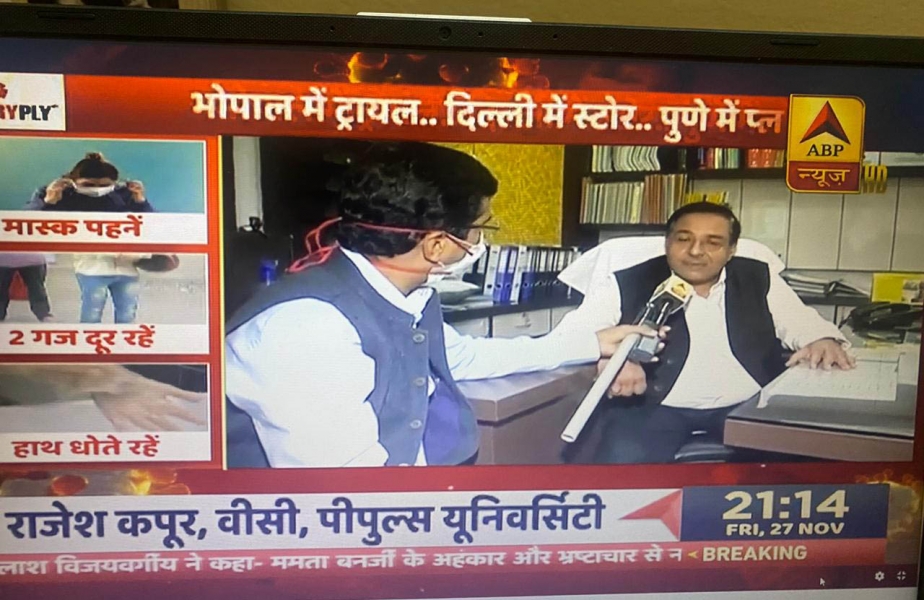
Phase-III Clinical Trials begins at People’s University, Bhopal
COVAXIN TM – India’s First indigenous COVID-19 Vaccine by Bharat Biotech is developed in collaboration with the Indian Council of Medical Research (ICMR) – National Institute of Virology (NIV). The indigenous, in activated vaccine is developed and manufactured in Bharat Biotech’s BSL-3 (Bio Safety Level 3) high containment facility. COVAXIN can be stored at temperatures ranging from 2-8 degrees.
The vaccine received DCGI approval for Phase I & II Human clinical Trials of COVAXIN TM Bharat Biotech received DCGI approval for Phase III clinical trials in 26,000 participants in over 25 centers across India.
Coronavirus vaccine phase III Clinical Trial
As part of the trial application, a dose of 0.5 ml would be given on day 0 and on day 28 intramuscular.
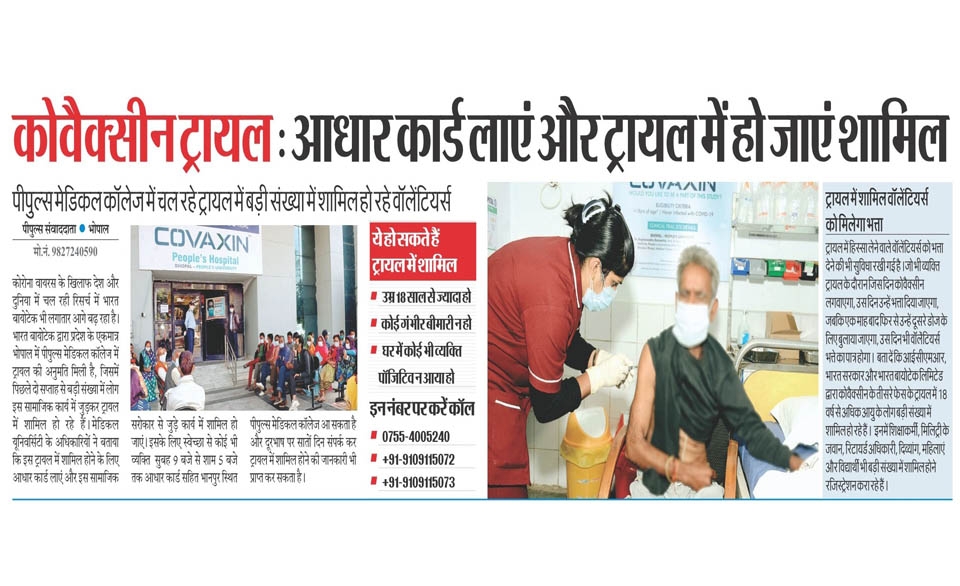
The phase – III randomised double-blind placebo-controlled multi-centre trial would cover around 28,500 subjects aged 18 years and above. it would be conducted in around 25 sites across 10 states.
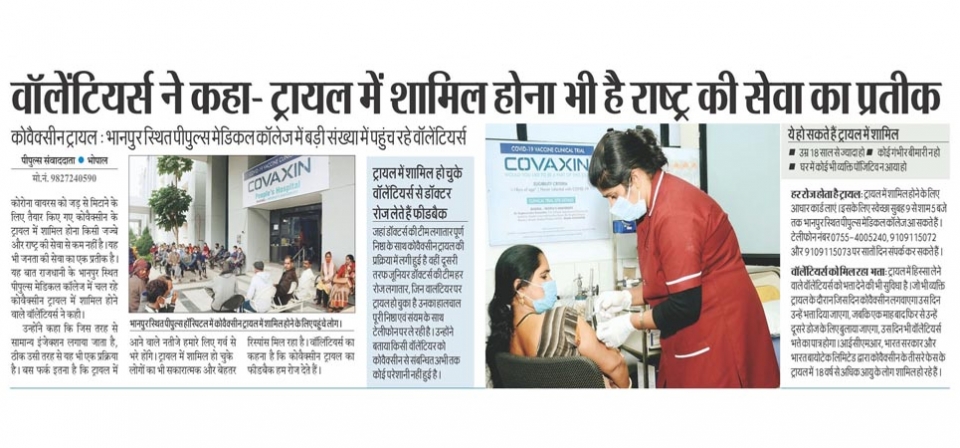
People’s College of Medical Science & RC, People’s University has initiated this trial in an estimated 2000 volunteers. It is the only Medical College from Madhya Pradesh and Chhattisgarh to be selected for this trial.